SAFE Interreg Project
A cross-border project to make medical implants safer and more reliable.
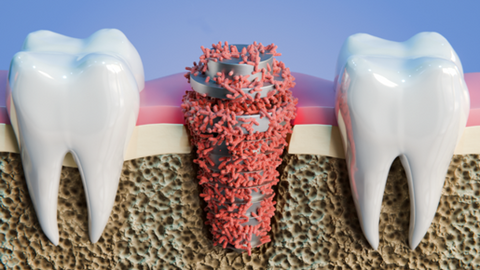
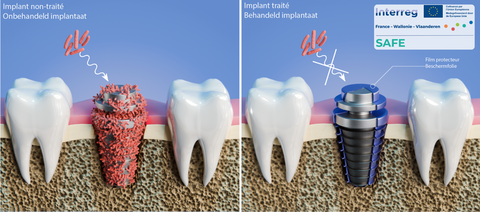
The SAFE project aims to develop plasma processes to treat the surfaces of medical implants and thus limit the risks of infection and inflammation. This project, funded by the Interreg France-Wallonie-Vlaanderen program, meets the objective of making the cross-border territory a region in which cutting-edge technologies are developed. This objective is also being pursued in the health and social fields, as the SAFE project aims to do.
The objectives of the SAFE project:
- Innovate and develop innovative technology using plasma processes.
- Improve the living conditions of the population in need of medical implants and strengthen their health protection.
- Make these innovative solutions available on the cross-border territory
- Foster cross-border cooperation to share expertise and best practices.
The SAFE project, thanks to the cooperation of several partners spread across France, Flanders and Wallonia, is helping to propel the cross-border territory covered by Interreg France-Wallonie-Vlaanderen to the forefront. While the aim is to innovate, the project does not lose sight of the practical application of its results, for a better quality of life for people using medical implants. In short, it's an innovative project at the service of the population!
Also visit LinkedIn or Facebook to make sure you don't miss any SAFE news. The project has launched the first edition of its bi-annual newsletter.


